Public urged to return Boots paracetamol due to mislabelled packs
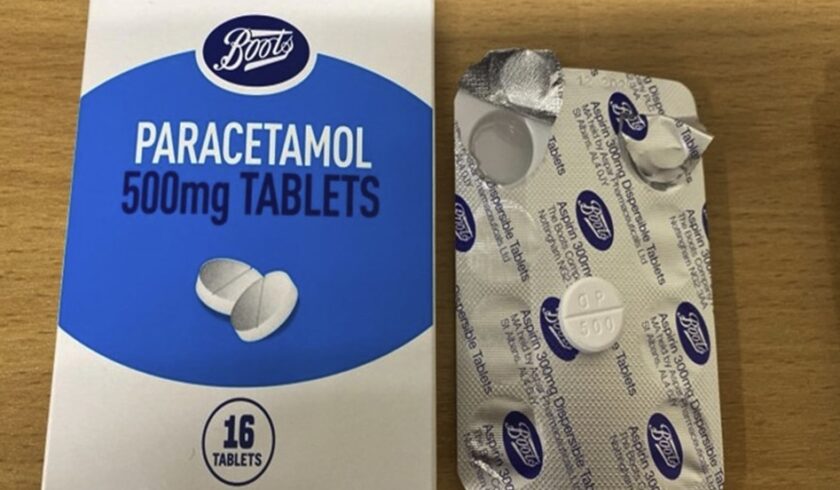
A batch of Boots Paracetamol 500mg Tablets has been recalled due to a packaging error that could lead to confusion over the medication, the Medicines and Healthcare products Regulatory Agency (MHRA) has announced.
The affected batch (241005) contains the correct paracetamol tablets, but the foil blister inside the packaging is incorrectly labelled as ‘Aspirin 300mg Dispersible Tablets.’
While the tablets themselves are confirmed to be paracetamol, the MHRA is urging customers to return the product to Boots stores for a full refund.
The recall affects 119,964 packs of Boots Paracetamol 500mg (16s), which have an expiry date of December 2029.
Customers, including carers, are advised to check the batch number on the bottom of the box.
If their pack is from the affected batch, they should stop using it and return it, even if they are aware of the error, as the mislabelling could lead to confusion and potential dosing mistakes.
Dr Stephanie Millican, MHRA Deputy Director Benefit Risk Evaluation, said: “Patient safety is always our priority. It is vitally important that you check the packaging of your Boots Paracetamol 500mg Tablets 16s, and if the batch number is 241005, you should stop using the product and return it to a Boots store for a full refund.”
The Boots Company PLC and the supplier, Aspar Pharmaceuticals Limited, have confirmed that the tablets are paracetamol and not aspirin and have launched an investigation into how the error occurred.
Anyone who is unsure whether they have purchased the affected batch or who experiences any side effects after taking Boots Paracetamol 500mg Tablets is advised to seek advice from a healthcare professional.
Suspected adverse reactions should be reported via the MHRA’s Yellow Card scheme.
Spotted something? Got a story? Send a Facebook Message | A direct message on Twitter | Email: [email protected] Latest News








